🇫🇷 For the French version of this article, click here
Hydrogen, a molecule with a myriad of applications, plays a pivotal economic role across various domains, ranging from ammonia production to fuels desulfurization, in addition to its presence in the agri-food industry, metallurgy, and space exploration. Its potential as a clean energy carrier has been gaining considerable traction in the battle against climate change and the transition toward a more sustainable economy.
Despite its contemporary association with innovation and modernity, the history of hydrogen stretches back far further than one might assume. This molecule, discovered over five centuries ago, has a history more intricate and unpredictable than initially thought.
This text is a transcription of a presentation given during the « Joining Forces on Hydrogen » congress organized by the Belgian Hydrogen Council on October 16, 2023. The complete set of slides can be downloaded here.
The genesis of hydrogen: discovery amidst alchemical enigma
Hydrogen (H2) is the tiniest and most enigmatic molecule in the realm of chemistry. Its existence is primarily attributed to the artifices of chemistry, as hydrogen, though it can be found naturally in certain geological formations, often necessitates synthesis.
In fact, it was through the purest of serendipities that hydrogen was first observed over five centuries ago by Paracelsus, an alchemist-philosopher, while he was engrossed in his alchemical experiments and esoteric quests. During these endeavors, Paracelsus noted the creation of an elusive gas, devoid of any discernible odor or coloration, when vitriol (the old name for sulfuric acid) met iron. However, even though he was the first to glimpse hydrogen and record it in his writings, Paracelsus could not fathom the nature of this gas or its composition (Figure 1).
Working with gases, these invisible and elusive components, was no straightforward task at that time. It would take more than a century before the brilliant Irish chemist and physicist, Robert Boyle, succeeded in collecting this novel gas in a small vial, born from the interaction of acid and metals. Nevertheless, the mystery endured, as Boyle could not differentiate hydrogen from the surrounding air. Year after year, the scientists of that era would emphasize that this gas, still lacking a known structure and a name, could ignite and even explode upon contact with a flame, releasing tremendous energy. It wasn’t until the Age of Enlightenment and the abandonment of the old alchemical dream that the French chemist Antoine Lavoisier, delving into the depths of modern chemistry, finally took an interest in this flammable gas that had piqued the curiosity of scholars for over two centuries. In 1783, he christened it « hydrogen » after observing that this gas produced water when reacting with oxygen.
Hydrogen, the magical breath of the past, was thus acknowledged as an artificial molecule, producible through chemical reactions. By the turn of the 18th century, hydrogen had a name, a well-established origin, and an identified structure. The properties of the molecule, some of which had already been highlighted by alchemists of yore, continued to evolve with the advancement of knowledge and modern chemical techniques. It became evident that hydrogen was of low density, much lighter than the surrounding air. It could be used to inflate balloons and airships. In the presence of oxygen, within a specially designed enclosure, it could generate energy in the form of electricity and water. Even Jules Verne, in his 1875 novel « The Mysterious Island, » dreamt of hydrogen and its interaction with oxygen, envisioning it as « an inexhaustible source of heat and light, of an intensity that coal could never match. One day, the holds of steamers and the tenders of locomotives, instead of coal, will be loaded with these compressed gases, which will burn in furnaces with enormous calorific power. »

Figure 1. The early moments in the history of hydrogen: a period spanning the 16th to the 19th centuries.
The production of hydrogen, aside from the traditional method based on the interaction between acids and metals, was already a tangible reality. In this pivotal year of 1800, two distinguished British scientists, William Nicholson and Anthony Carlisle, achieved a remarkable scientific feat. Just a few days after the birth of the very first electric cell, a product of Alessandro Volta’s genius, these scholars succeeded in developing the sophisticated process of water electrolysis. This ingenious method allowed the decomposition of water into its fundamental components, namely oxygen and hydrogen, by passing an electric current between two thin metal plates immersed in water. It is truly striking to contemplate the irony of the situation. At the time, at the turn of the 19th century, this discovery seemed firmly entrenched in scientific knowledge. However, at the dawn of the 21st century, within a context of escalating environmental and energy concerns, it would re-emerge as a pivotal subject of study for contemporary researchers in pursuit of a « green » and virtuous source of hydrogen.
From zeppelins to space exploration: a century of commercial abundance
As the initial investigations had highlighted the energetic qualities and density of hydrogen, the early 20th century marked a turning point in the history of hydrogen applications, a shift foreseen by Paul Sabatier as early as 1897. Hydrogen, owing to its highly reactive nature, demonstrated its ability to interact with various molecules. When used to treat liquid vegetable oils, they were transformed into a solid substance, giving rise to margarine.
When hydrogen underwent a reaction with nitrogen in reactors specially designed with catalysts, it converted into ammonia (NH3), thus becoming a crucial element in the production of nitrogen fertilizers. Furthermore, hydrogen had the capacity to metamorphose into methane or methanol when it met carbon dioxide (CO2) or carbon monoxide (CO). These processes, initiated well before the outbreak of the Second World War, continue to be employed to this day. They represent, moreover, the predominant industrial applications of hydrogen, alongside desulfurization, which involves reducing the sulfur compound content in fuels for transportation, a technique that was scaled up in 1949 (Figure 2).
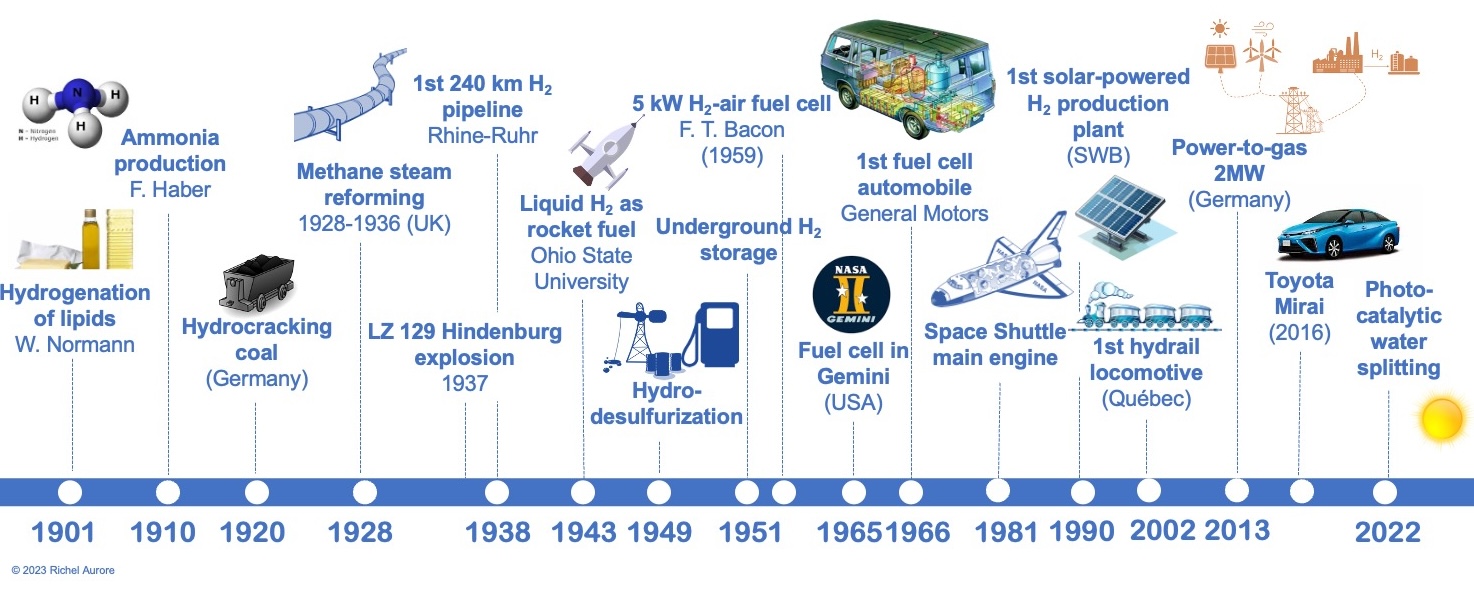
Figure 2. Contemporary history of hydrogen: from the early 20th century to the present day.
In response to the relentless growth of industrial demand, water electrolysis, although already mastered at the time, unfortunately proved to be inefficient. The required energy demands were excessive, while the yields obtained remained inadequate. Faced with this challenge, eminent researchers of the era turned to the valuable fossil resources, notably coal and natural gas.
The transformation of coal into hydrogen, commonly known as « gasification, » as well as the process of converting methane into hydrogen, known as « steam reforming, » were deployed on a substantial industrial scale in the 1920s and 1930s. It is crucial to note, however, that these methods, while practical and successful, suffer from a significant environmental drawback. They generate substantial quantities of carbon compounds, including carbon dioxide, which classifies them as environmentally impactful methods. Nonetheless, it is worth noting that these traditional techniques remain predominant to this day, accounting for over 98% of global hydrogen production.
Following the Second World War, hydrogen found itself in the spotlight regarding its use in the field of mobility. The optimization of fuel cells, these remarkable devices discovered as early as the 1840s capable of generating electricity when hydrogen encounters oxygen, enabled the introduction of the very first automotive van by General Motors in 1966 and the Toyota Mirai in 2016.
The use of hydrogen to power trains, buses, and even light vehicles has also been subject to thorough evaluation over the past three decades, with varying results. Meanwhile, hydrogen enjoyed a heyday in the American space exploration, from the Gemini program to the missions of the latest space shuttles. Fuel cells aboard played an essential role in providing the necessary energy inputs to the crews and a sufficient water supply.
A Belated Academic Exploration Focusing on the Link Between Hydrogen and Nuclear Energy
Throughout these five centuries marked by intense research and discoveries aligned with major industrial advancements, the scientific contributions documented by academics truly began to stand out only from the 1970s onward. Before this period, apart from the erudite writings of great scholars from the 17th to the 19th centuries, the scientific literature devoted to hydrogen was not particularly abundant, in contrast to other scientific fields that garnered the enthusiasm of university teams from the early 20th century.
Thus, it wasn’t until 1972 and the publication of the concept of a « hydrogen economy » in the prestigious journal Science that academic institutions began to contemplate hydrogen as a molecule inherently endowed with the potential to extend beyond the already ongoing commercial applications (Figure 3).
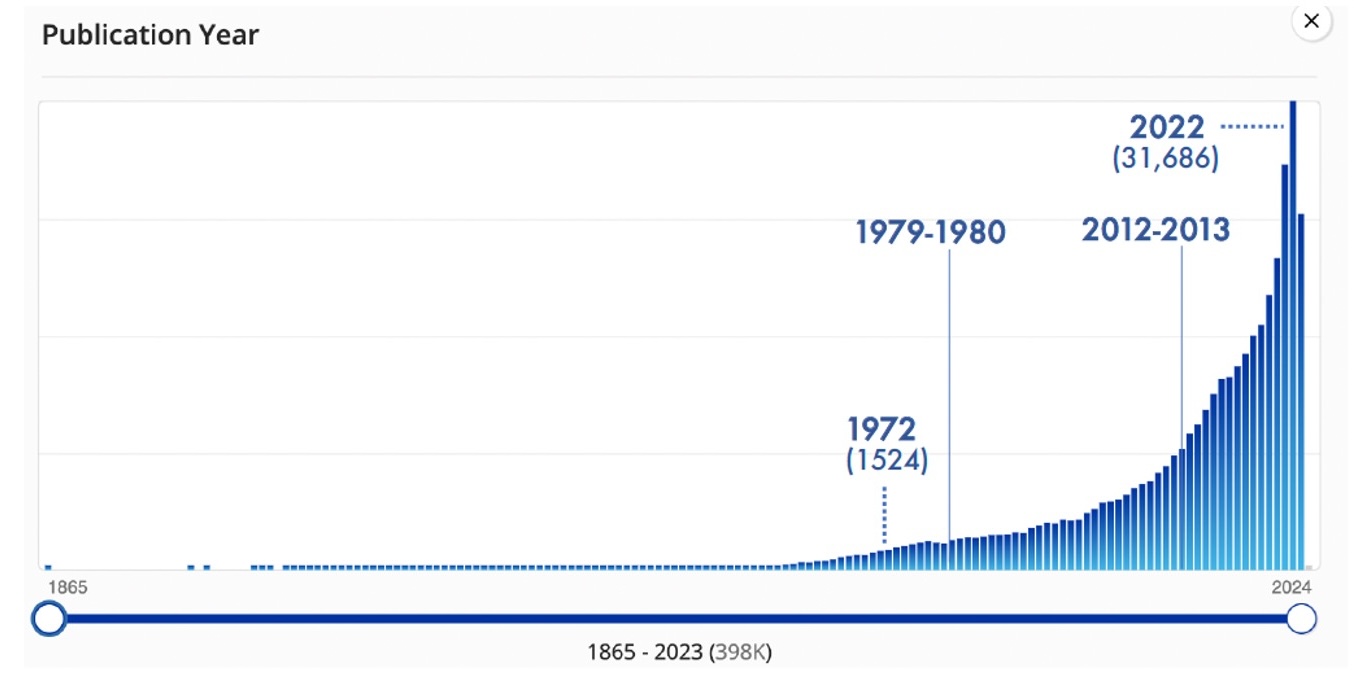
Figure 3. Evolution of the number of scientific articles published from the mid-19th century to the present day related to hydrogen (source: SciFinder, database consulted in October 2023).
Within the pages of the prestigious journal Science, Professor J. O’M. Bockris put forward the fascinating perspective that hydrogen could serve as a carrier to transport the energy generated by nuclear reactors to end-users. In the initial formulations of hydrogen’s energy roles concerning the future, quite surprisingly, hydrogen and atomic energy were intimately intertwined. Bockris’s proposal revolved around the establishment of large offshore platforms in the open sea, where water electrolysis would be performed using electricity supplied by nuclear reactors. The hydrogen thus produced would then be transported to the mainland, where stations would handle its distribution to industries or individuals. Bockris proved to be a visionary, emphasizing that due to the continuous operation of nuclear reactors, hydrogen production would not be subject to interruptions. However, he was aware that public acceptance of his idea could be hindered by several factors, including the prevailing conservatism favoring fossil fuels, the lack of training and education in the new electrochemistry-related technologies, and public concerns about the explosive nature of hydrogen.
Bockris was convinced that these obstacles could be quickly overcome if mobility, especially automobiles, became the most obvious illustration of his concept. Of course, all of this was published before the Chernobyl accident, before the public and political authorities definitively altered their perspective on nuclear energy. What a striking paradox to observe that the persistent idea of offshore hydrogen production is still deeply rooted in future visions. Today, however, intermittent renewable energies, particularly large offshore wind turbines, have supplanted the nuclear reactors that were once widely praised by Bockris and his contemporaries.
The academic research structured in three distinct periods
If we closely examine the intricacies of scientific research since the publication of Bockris’s work in 1972, we can clearly discern an evolution in three distinct phases (Figure 3). In the first act, spanning from 1972 to 1979, the foundations of thinking about the hydrogen economy and its place in the energy landscape of that era begin to emerge. Scientific publications are relatively scarce, in line with the knowledge dissemination practices of that bygone period. Between 1980 and 2012, research on hydrogen advances steadily, although there is a noticeable deceleration and gradual stagnation. Conversely, since 2013, there has been a spectacular upsurge in writings devoted to hydrogen. This renaissance is explained, on the one hand, by a shift in publication practices that emphasize the famous adage « publish or perish, » and on the other hand, by a renewed interest in hydrogen, propelling it to the forefront of energy transition strategies.
Throughout these five decades of research, academics have delved into every facet of the hydrogen value chain, from production to applications, encompassing storage, distribution, as well as the techno-economic and environmental aspects (Figure 4). The sections devoted to hydrogen production methods and its applications, whether as a reactant or an energy carrier, together account for nearly three-quarters of the scientific output to date. It is noteworthy that, despite its discovery in 1800, water electrolysis only began to be explored in detail as a hydrogen production method in the 1970s. In parallel, traditional methods of production from fossil resources continue to be studied by researchers, including to this day.
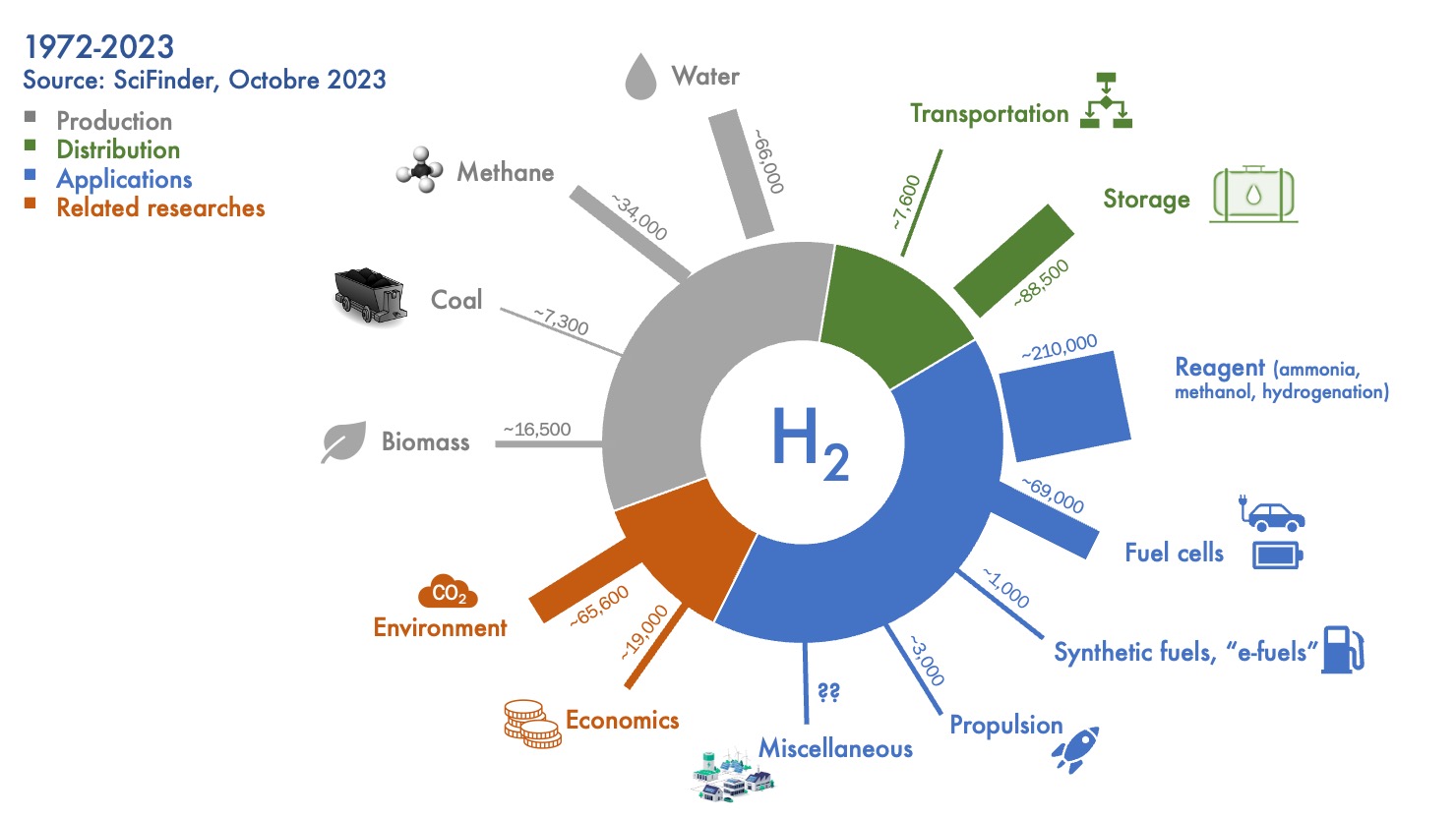
Figure 4. Distribution of hydrogen-related publications based on research topics across the entire value chain. The numbers provided for each research area represent the total number of publications recorded over the past fifty years.
The 1970s – The Birth of Academic Hydrogen Research
Following the publication of Bockris’s work, a plethora of holistic studies took flight, delving deeply into the complexity of the hydrogen economy concept. During this era marked by the energy shocks of 1973 and 1979, hydrogen emerged as a potential messenger of resilient energy. Strangely, the environmental implications of this promising avenue were not at the forefront of researchers’ thoughts at that time; they were primarily focused on the production and application aspects of hydrogen, considered undeniable priorities. It was at the dawn of this period, marked by the establishment of the International Energy Agency (IEA) and the International Association for Hydrogen Energy (IAHE), that research investments primarily turned towards optimizing hydrogen production processes. Among these paths, the integration of nuclear energy as a central pillar of production continued to haunt the minds. In fact, more than half of the works published during this decade were devoted to the union of hydrogen and nuclear energy, forging an indissoluble link between these two domains.
From 1980 to 2012: Stagnation and Exploratory Research
After the energy crisis of 1979, the urgency to search for alternative energy sources became less pressing. However, the quest for a sustainable path persisted, driven by an initial desire to improve mobility while respecting the environment and supported by some industrial pioneers. The association between hydrogen and nuclear energy, once solid, abruptly faded following the Chernobyl disaster in 1986. The gradual realization of the role played by greenhouse gases and climate change gradually pushed universities toward renewable energy sources such as solar and wind energy. Hydrogen thus positioned itself as a solution for storing and distributing these two intermittent renewable energies from the early 2000s. During this period, hydrogen research addressed broader issues, including the storage of this molecule and its transportation. This period was also marked by numerous works on fuel cells for the transportation sector and the first environmental assessments of the hydrogen value chain.
Since 2013: An Explosion of Research
Over the past decade, research publications on hydrogen have experienced a spectacular upsurge. The gradually increasing media coverage of the IPCC’s work, combined with a growing awareness of the climate crisis, unlocked substantial funding, breathing new life into hydrogen research. Hydrogen is now perceived as an energy source that can be produced locally or nationally, reducing dependence on foreign countries, and offering clean, local energy that seems, albeit falsely, detached from geopolitical implications. Hailed as an abundant, affordable, widely accessible, and environmentally friendly energy carrier, hydrogen is inseparable from water electrolysis powered by renewable energy. Beyond this vision, research has flourished in various directions, with notable progress in improving materials for electrolyzers, innovating membranes, exploring new approaches for storage and transportation, studying materials for modifying existing gas networks, conducting in-depth techno-economic analyses, producing synthetic fuels and electro-fuels, exploring enhanced fuel cells, and investigating the links between hydrogen and metallurgy.
However, it appears that research, despite its expansion, is still searching for its true direction. It sometimes seems to react to external influences, whether political, economic, or ideological, and sometimes suffers not to be multidisciplinary. It is still in need of coherence and stability that would allow it to fully unfold its potential.
What Should We Remember?
Hydrogen, once touted as « the » molecule of the future, has a much deeper history than its recent accolades. Known for over five centuries, it only captured the attention of universities in the 1970s when the need to design resilient energy systems became critical. A dive into research data, reflected by the number of publications, reveals three distinct eras that have marked the history of hydrogen research. Each of these periods, influenced by well-defined external events, gave rise to distinct, sometimes divergent research directions. By delving into these past narratives related to hydrogen, researchers could reshape the current research landscape. This endeavor is crucial to prevent hydrogen from being reduced to a passing phenomenon, despite massive environmental, political, and economic support. Hydrogen deserves to be a lasting player in the future energy landscape, and its history can illuminate this path.
More information?
Do not hesitate to contact me via the following email address: a.richel@uliege.be or via the form available by clicking here.



